Contained use of genetically modified organisms
The Gene Technology Act regulates the contained use of genetically modified micro-organisms, plants and animals in Finland. As defined by the Act, contained use means any activity in which organisms are genetically modified or in which such GMOs are cultured, stored, transported, destroyed or disposed of or used in any other way, and for which specific containment measures are used to limit their contact with the general population and the environment and to provide a high level of safety for the general population and the environment. Contained use is often carried out as basic research of biology or medicine in laboratories in universities or research institutes.
Every operator using GMOs has certain duties laid down by the Act. The operator must make in writing a risk assessment of the use of each GMO in advance and be careful when using them. The operator has a duty to obtain information on the properties of the GMOs and to keep a record of the contained use. Also, the operator has a duty to submit relevant notifications of or applications for the contained use to the Board for Gene Technology and to update the documents as specified by the Act.
The Board collects charges from operators for processing notifications and applications in accordance with Government Decree on Chargeable Performances under the Gene Technology Act.
Notification procedure in a nutshell
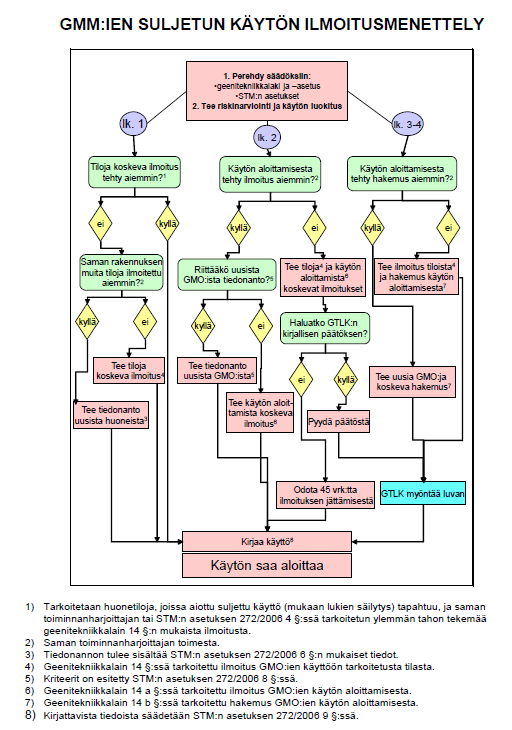
Flow chart of the notification procedure (in Finnish) as a PDF file.
